Why Does Potassium Chloride Have a High Melting Point
The solid dissolves readily in water and its solution s have a salt -like taste. It dissolves in water does not conduct electricity when solid but does when melted and it has a high melting point 804 C.
Potassium Chloride Kcl Properties Uses Side Effects
Conpounds with ionic bonds have high melting points.

. Why does strontium chloride have a high melting point. Aluminum chloride molecules are attracted to each other by van der Waals forces. The molar mass of KCl is 745513 gramsmol.
Why does potassium chloride have a high melting point. Since more energy is required to break the bonds the melting point of calcium will be higher than that of potassium. All ionic compounds have high melting points for this reason.
Since more energy is required to break the bonds the melting point of calcium will be higher than that of potassium. This is because sodium chloride is an ionic compound and aluminum chloride is a simple molecular compound. As the ionic lattice contains such a large number of ions a lot of energy is needed to overcome this ionic bonding so ionic compounds have high melting and boiling points.
Why does potassium chloride have a high melting point. February 16 2021. Sodium chloride and potassium chloride are ionic compoundsBoth are solids and their cations and anions are in a closely packed structure.
This ion has a greater charge than Li and so forms stronger attractions. Ionic compounds are held together by electrostatic forces between the oppositely charged ions. Which of the following compounds would have have the lowest melting point.
This requires more heat energy to overcome. The melting and boiling points of potassium chloride are 1040 K and 1690 K respectively. It also has a giant lattice structure which means that it contains millions of strong ionic bonds.
What is the melting point of LICL. NaCl has a higher melting point 801C 1474F than A l 2 C l 6 193C 379F. Since the electrostatic forces of attraction between oppositely charged ions are.
In practice once calcium chloride and magnesium chloride touch ice or snow they immediately pick up water to form a strong brine emit heat to give added deicing effect create more water and form more brine. Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions. Lithium chloride is mainly used for the production of lithium metal by electrolysis of a LiClKCl melt at 450 C 842 F.
The key difference between sodium chloride and potassium chloride is that the electronegativity difference between K and Cl is higher than that of Na and Cl. Sodium chloride has a high melting point because of the strong electrostatic attraction between its positive and negative ions. This energy is provided in the high melting temperatureCaO has a higher melting point than LiCl due to the Ca 2 ion.
Why is the measured ph different to calculated ph How To Balance Cu HNO3 CuNO32 NO H2O balancing redox equations. The crystals of potassium chloride are made up of face-centred cubic FCC unit cells. This requires more heat energy to overcome.
Its density in the solid crystalline form is 1984 grams per cubic centimetre. Ionic compounds are held together by electrostatic forces between the oppositely charged ions. Magnesium oxide MgO and potassium chloride KCl are other ionic solids.
The more energy needed the higher the melting point. All ionic compounds have high melting points for this reason. Ionic substances all have high melting and boiling points.
Well if you compare it to methane whose molecules are almost the same size and weight it has a surprisingly high melting point and boiling point. It has a high melting point and boiling point as much energy is needed to break the many strong covalent bonds. Click card to see definition.
Which chloride has the highest melting point. Sodium chloride has a high melting and boiling point There are strong electrostatic attractions between the positive and negative ions and it takes a lot of heat energy to overcome them. These attractions are strong and so require a large amount of energy to break.
The melting and boiling points of molecular substances are determined largely by the intermolecular forces. Since there will be more delocalized electrons per atom throughout calcium than potassium the bond strength is stronger in calcium than potassium. Explaining melting points It takes a lot of energy to overcome the strong electrostatic forces of attraction between oppositely charged ions so ionic compounds have high melting and.
A lot of energy is needed to separate the atoms in diamond. These are group 1 metals which have the capability to make 1. Tap card to see definition.
Does KCL have a high melting point. Melting point of Chlorine is -101C. Explaining melting points It takes a lot of energy to overcome the strong electrostatic forces of attraction between oppositely charged ions so ionic compounds have high melting and boiling points.
As the ionic lattice contains such a large number of ions a lot of energy is needed to overcome this ionic bonding so ionic compounds have high melting and boiling points.

Properties Of Ionic Materials Activity
Difference Between Potassium Gluconate And Potassium Chloride Definition Chemical Structure And Properties Differences

Potassium Chloride Trading Www Brokermet Com
How Is Potassium Chloride An Iconic Bond Quora
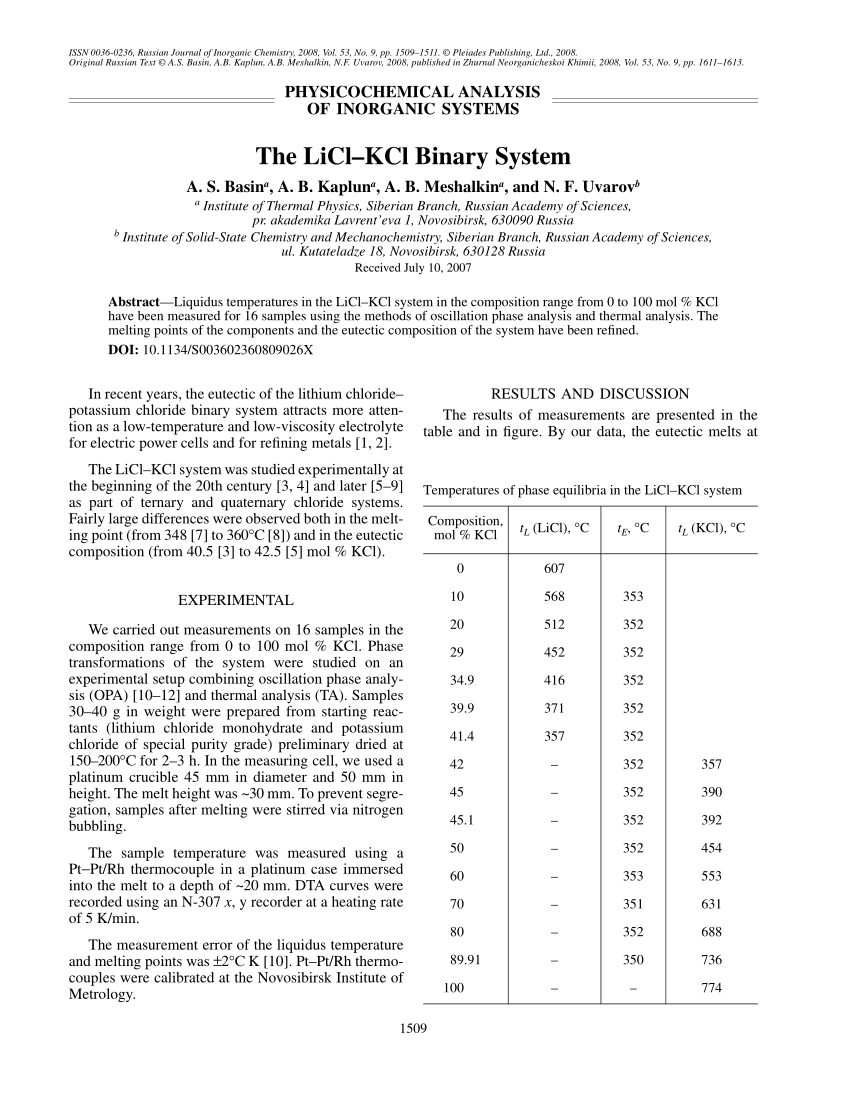
Pdf The Licl Kcl Binary System

Solved 1 A Potassium Chloride Is An Ionic Chloride With Chegg Com

Why Is The Melting Point Of Kcl Less Than Nacl Quora

Aqa Gcse 9 1 Chemistry For Combined Science Trilogy By Collins Issuu

Binary Phase Diagram Of Kcl And Nacl Liq Is A Molten Solution Download Scientific Diagram

Solved Part 1 Why Does Bas Have A Much Higher Melting Point Chegg Com

Solved Explain For Each Observation With Appropriate Atomic Chegg Com
Why Is The Melting Point Of Kcl Less Than Nacl Quora

No Comp Book Question Write Down Your Homework Ppt Download
Potassium Chloride Cas 7447 40 7

Binary Phase Diagram Of Kcl And Nacl Liq Is A Molten Solution Download Scientific Diagram